Early patient identification is critical to outcomes in non-Hodgkin lymphoma treatment1
Know when it’s time to consider YESCARTA2
YESCARTA is indicated for the treatment of adult patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL) after ≥2 lines of systemic therapy, including:
- Diffuse large B-cell lymphoma (DLBCL) not otherwise specified
- Primary mediastinal large B-cell lymphoma (PMBCL)
- High grade B-cell lymphoma
- DLBCL arising from follicular lymphoma
Limitations of Use: YESCARTA is not indicated for the treatment of patients with primary central nervous system lymphoma.
Learn more about YESCARTA for a different indication
A closer look at who may be appropriate for YESCARTA CAR T therapy
Learn why these actual patients were treated with YESCARTA. Featured patients are sponsored by Kite. Not all patients will experience the same results, and side effects may vary.
†Patient ages are reflective of the age they were at time of interview.
YESCARTA treatment: personalized cell therapy built on close collaboration
Your role is essential in the YESCARTA treatment journey
Whether it’s before, during, or after treatment, the collaboration between the primary hematologist/oncologist and the YESCARTA Authorized Treatment Center (ATC) is critical to help give your patients the best possible care.
Before
Treatment
(PRIMARY HEM/ONC)
During
Treatment
(ATCs)
After
Treatment
(PRIMARY HEM/ONC)
STEP 1

PROACTIVE PATIENT IDENTIFICATION
PROACTIVE PATIENT IDENTIFICATION IS CRITICAL TO OUTCOMES
While YESCARTA is indicated after ≥2 lines of systemic therapy, you can start identifying and educating your patients proactively in order to accelerate time between potential second-line failure and CAR T treatment.2
STEP 2

EARLY SPECIALIST CONSULTATION
CONSULT WITH A CAR T SPECIALIST AT AN AUTHORIZED TREATMENT CENTER THAT MEETS YOUR PATIENT’S NEEDS
Early consultation helps determine if YESCARTA is right for your patient and maximizes their chance at receiving therapy. The optimal time for a consultation may be as soon as first relapse to help ensure optimal access to all available treatment options.1 Consider telemedicine consultations, which may be covered by a patient’s insurance. Always check with the patient’s plan to confirm coverage.
YESCARTA is available only at Authorized Treatment Centers with specialized CAR T healthcare teams trained on how to administer, monitor, and care for patients.2 Choice of an Authorized Treatment Center is within the sole discretion of the treating physician and patient. Kite does not endorse any individual treatment sites.
STEP 3

TIMELY ACCESS
MAXIMIZE YOUR PATIENT’S CHANCE AT RECEIVING YESCARTA
As soon as second-line treatment failure or relapse, patients can be evaluated for CAR T therapy at an Authorized Treatment Center. Positioning CAR T treatment later in the disease course runs the risk of patients becoming too frail as the result of advanced disease or side effects of prior treatments.1 Once assessed by the CAR T team, your qualified third-line patients can begin YESCARTA therapy.
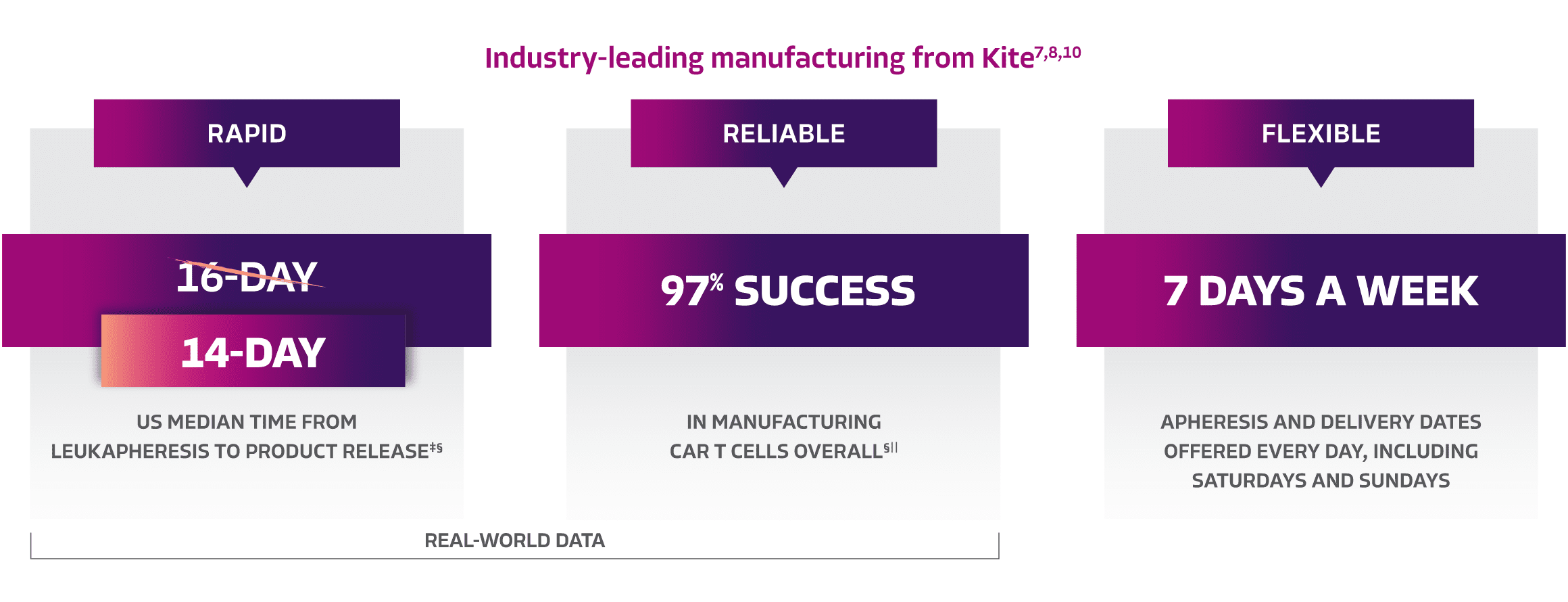
KITE: Redefining CAR T therapy with best-in-class manufacturing, ensuring rapid and reliable treatment delivery when patients need it most7-9
‡Time from product release to treatment center may vary. Manufacturing data are reflective of the entire YESCARTA product line.7,8
§Real-world US manufacturing data since Q1 2024.8
||Real-world manufacturing data as of January 2024.7
¶In LBCL. US claims data as of March 2024.11
#YESCARTA needs to be administered at a certified healthcare facility,1 with setting of care based on HCP discretion.
**Global commercial and clinical trial data in patients across the entire YESCARTA product line as of September 2023.3
1L=first-line; 2L=second line; ASCT=autologous stem cell transplant; BEAM=carmustine, etoposide, cytarabine, melphalan; CAR T=chimeric antigen receptor T cell; CRS=cytokine release syndrome; CT=computed tomography; ECOG PS=Eastern Cooperative Oncology Group performance status; EPOCH=etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride; HEM/ONC=hematologist/oncologist; ICU=intensive care unit; IgG=immunoglobulin G; IVIG=intravenous immunoglobulin; MRI=magnetic resonance imaging; PET=positron emission tomography; PFT=pulmonary function test; PMBCL=primary mediastinal large B-cell lymphoma; R-CHOP=rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-GDP=rituximab, gemcitabine, dexamethasone, cisplatin; R-ICE=rituximab, ifosfamide, carboplatin, etoposide phosphate; SVC=superior vena cava; TFL=transformed follicular lymphoma.
IMPORTANT SAFETY INFORMATION
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, and SECONDARY HEMATOLOGICAL MALIGNANCIES
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving YESCARTA. Do not administer YESCARTA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids.
- Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving YESCARTA, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with YESCARTA. Provide supportive care and/or corticosteroids as needed.
- T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA.
- YESCARTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS.
CYTOKINE RELEASE SYNDROME (CRS)
CRS, including fatal or life-threatening reactions, occurred following treatment with YESCARTA. CRS occurred in 90% (379/422) of patients with non-Hodgkin lymphoma (NHL), including ≥ Grade 3 CRS in 9%. CRS occurred in 93% (256/276) of patients with large B-cell lymphoma (LBCL), including ≥ Grade 3 in 9%. Among patients with LBCL who died after receiving YESCARTA, 4 had ongoing CRS events at the time of death. For patients with LBCL in ZUMA-1, the median time to onset of CRS was 2 days following infusion (range: 1-12 days) and the median duration was 7 days (range: 2-58 days). For patients with LBCL in ZUMA-7, the median time to onset of CRS was 3 days following infusion (range: 1-10 days) and the median duration was 7 days (range: 2-43 days).
CRS occurred in 84% (123/146) of patients with indolent non-Hodgkin lymphoma (iNHL) in ZUMA-5, including ≥ Grade 3 CRS in 8%. Among patients with iNHL who died after receiving YESCARTA, 1 patient had an ongoing CRS event at the time of death. The median time to onset of CRS was 4 days (range: 1-20 days) and median duration was 6 days (range: 1-27 days) for patients with iNHL.
Key manifestations of CRS (≥ 10%) in all patients combined included fever (85%), hypotension (40%), tachycardia (32%), chills (22%), hypoxia (20%), headache (15%), and fatigue (12%). Serious events that may be associated with CRS include, cardiac arrhythmias (including atrial fibrillation and ventricular tachycardia), renal insufficiency, cardiac failure, respiratory failure, cardiac arrest, capillary leak syndrome, multi-organ failure, and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS).
The impact of tocilizumab and/or corticosteroids on the incidence and severity of CRS was assessed in 2 subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received tocilizumab and/or corticosteroids for ongoing Grade 1 events, CRS occurred in 93% (38/41), including 2% (1/41) with Grade 3 CRS; no patients experienced a Grade 4 or 5 event. The median time to onset of CRS was 2 days (range: 1-8 days) and the median duration of CRS was 7 days (range: 2-16 days). Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA. Thirty-one of the 39 patients (79%) developed CRS and were managed with tocilizumab and/or therapeutic doses of corticosteroids with no patients developing ≥ Grade 3 CRS. The median time to onset of CRS was 5 days (range: 1-15 days) and the median duration of CRS was 4 days (range: 1-10 days). Although there is no known mechanistic explanation, consider the risk and benefits of prophylactic corticosteroids in the context of pre-existing comorbidities for the individual patient and the potential for the risk of Grade 4 and prolonged neurologic toxicities.
Ensure that 2 doses of tocilizumab are available prior to YESCARTA infusion. Monitor patients for signs and symptoms of CRS following infusion at least daily for 7 days at the certified healthcare facility, and for 4 weeks thereafter. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated.
NEUROLOGIC TOXICITIES
Neurologic toxicities including immune effector cell-associated neurotoxicity syndrome (ICANS) that were fatal or life-threatening occurred following treatment with YESCARTA. Neurologic toxicities occurred in 78% (330/422) of patients with NHL receiving YESCARTA, including ≥ Grade 3 in 25%.
Neurologic toxicities occurred in 87% (94/108) of patients with LBCL in ZUMA-1, including ≥ Grade 3 in 31% and in 74% (124/168) of patients in ZUMA-7 including ≥ Grade 3 in 25%. The median time to onset was 4 days (range: 1-43 days) and the median duration was 17 days for patients with LBCL in ZUMA-1. The median time to onset for neurologic toxicity was 5 days (range: 1-133 days) and median duration was 15 days in patients with LBCL in ZUMA-7. Neurologic toxicities occurred in 77% (112/146) of patients with iNHL, including ≥ Grade 3 in 21%. The median time to onset was 6 days (range: 1-79 days) and the median duration was 16 days. Ninety-eight percent of all neurologic toxicities in patients with LBCL and 99% of all neurologic toxicities in patients with iNHL occurred within the first 8 weeks of YESCARTA infusion. Neurologic toxicities occurred within the first 7 days of infusion in 87% of affected patients with LBCL and 74% of affected patients with iNHL.
The most common neurologic toxicities (≥ 10%) in all patients combined included encephalopathy (50%), headache (43%), tremor (29%), dizziness (21%), aphasia (17%), delirium (15%), and insomnia (10%). Prolonged encephalopathy lasting up to 173 days was noted. Serious events, including aphasia, leukoencephalopathy, dysarthria, lethargy, and seizures occurred. Fatal and serious cases of cerebral edema and encephalopathy, including late-onset encephalopathy, have occurred.
The impact of tocilizumab and/or corticosteroids on the incidence and severity of neurologic toxicities was assessed in 2 subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received corticosteroids at the onset of Grade 1 toxicities, neurologic toxicities occurred in 78% (32/41) and 20% (8/41) had Grade 3 neurologic toxicities; no patients experienced a Grade 4 or 5 event. The median time to onset of neurologic toxicities was 6 days (range: 1-93 days) with a median duration of 8 days (range: 1-144 days). Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA. Of those patients, 85% (33/39) developed neurologic toxicities; 8% (3/39) developed Grade 3, and 5% (2/39) developed Grade 4 neurologic toxicities. The median time to onset of neurologic toxicities was 6 days (range: 1-274 days) with a median duration of 12 days (range: 1-107 days). Prophylactic corticosteroids for management of CRS and neurologic toxicities may result in higher grade of neurologic toxicities or prolongation of neurologic toxicities, delay the onset, and decrease the duration of CRS.
Monitor patients for signs and symptoms of neurologic toxicities following infusion at least daily for 7 days at the certified healthcare facility, and for 4 weeks thereafter, and treat promptly.
REMS
Because of the risk of CRS and neurologic toxicities, YESCARTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS. The required components of the YESCARTA and TECARTUS REMS are:
- Healthcare facilities that dispense and administer YESCARTA must be enrolled and comply with the REMS requirements. Certified healthcare facilities must have on-site, immediate access to tocilizumab, and ensure that a minimum of 2 doses of tocilizumab are available for each patient for infusion within 2 hours after YESCARTA infusion, if needed for treatment of CRS.
Further information is available at www.YescartaTecartusREMS.com or 1-844-454-KITE (5483).
HYPERSENSITIVITY REACTIONS
Allergic reactions may occur with the infusion of YESCARTA. Serious hypersensitivity reactions, including anaphylaxis, may be due to dimethyl sulfoxide (DMSO) or residual gentamicin in YESCARTA.
SERIOUS INFECTIONS
Severe or life-threatening infections occurred after YESCARTA infusion. Infections (all grades) occurred in 45% of patients with NHL. Grade 3 or higher infections occurred in 17% of patients, including ≥ Grade 3 infections with an unspecified pathogen in 12%, bacterial infections in 5%, viral infections in 3%, and fungal infections in 1%. YESCARTA should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of infection before and after infusion and treat appropriately. Administer prophylactic antimicrobials according to local guidelines.
Febrile neutropenia was observed in 36% of patients with NHL and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
In immunosuppressed patients, including those who have received YESCARTA, life-threatening and fatal opportunistic infections including disseminated fungal infections (e.g., candida sepsis and aspergillus infections) and viral reactivation (e.g., human herpes virus-6 [HHV-6] encephalitis and JC virus progressive multifocal leukoencephalopathy [PML]) have been reported. The possibility of HHV-6 encephalitis and PML should be considered in immunosuppressed patients with neurologic events and appropriate diagnostic evaluations should be performed.
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, has occurred in patients treated with drugs directed against B cells, including YESCARTA. Perform screening for HBV, HCV, and HIV and management in accordance with clinical guidelines before collection of cells for manufacturing.
PROLONGED CYTOPENIAS
Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and YESCARTA infusion. Grade 3 or higher cytopenias not resolved by Day 30 following YESCARTA infusion occurred in 39% of all patients with NHL and included neutropenia (33%), thrombocytopenia (13%), and anemia (8%). Monitor blood counts after infusion.
HYPOGAMMAGLOBULINEMIA
B-cell aplasia and hypogammaglobulinemia can occur in patients receiving YESCARTA. Hypogammaglobulinemia was reported as an adverse reaction in 14% of all patients with NHL. Monitor immunoglobulin levels after treatment and manage using infection precautions,
antibiotic prophylaxis, and immunoglobulin replacement.
The safety of immunization with live viral vaccines during or following YESCARTA treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during YESCARTA treatment, and until immune recovery following treatment.
SECONDARY MALIGNANCIES
Patients treated with YESCARTA may develop secondary malignancies. T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA. Mature T cell malignancies, including CAR-positive tumors, may present as soon as weeks following infusion, and may include fatal outcomes.
Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
Due to the potential for neurologic events, including altered mental status or seizures, patients are at risk for altered or decreased consciousness or coordination in the 8 weeks following YESCARTA infusion. Advise patients to
refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 20%) in patients with LBCL in ZUMA-1 included CRS, fever, hypotension, encephalopathy, tachycardia, fatigue, headache, decreased appetite, chills, diarrhea, febrile neutropenia, infections with pathogen unspecified, nausea, hypoxia, tremor, cough, vomiting, dizziness, constipation, and cardiac arrhythmias.
INDICATIONMORE
YESCARTA® is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of:
- Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
Limitations of Use: YESCARTA is not indicated for the treatment of patients with primary central nervous system lymphoma.
Please see full Prescribing Information, including BOXED WARNING and Medication Guide.
Authorized Treatment Centers are independent facilities certified to dispense Kite CAR T therapies. Choice of an Authorized Treatment Center is within the sole discretion of the physician and patient. Kite does not endorse any individual treatment sites.
IMPORTANT SAFETY INFORMATION
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, and SECONDARY HEMATOLOGICAL MALIGNANCIES
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving YESCARTA. Do not administer YESCARTA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids.
- Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving YESCARTA, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with YESCARTA. Provide supportive care and/or corticosteroids as needed.
- T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA.
- YESCARTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS.
CYTOKINE RELEASE SYNDROME (CRS)
CRS, including fatal or life-threatening reactions, occurred following treatment with YESCARTA. CRS occurred in 90% (379/422) of patients with non-Hodgkin lymphoma (NHL), including ≥ Grade 3 CRS in 9%. CRS occurred in 93% (256/276) of patients with large B-cell lymphoma (LBCL), including ≥ Grade 3 in 9%. Among patients with LBCL who died after receiving YESCARTA, 4 had ongoing CRS events at the time of death. For patients with LBCL in ZUMA-1, the median time to onset of CRS was 2 days following infusion (range: 1-12 days) and the median duration was 7 days (range: 2-58 days). For patients with LBCL in ZUMA-7, the median time to onset of CRS was 3 days following infusion (range: 1-10 days) and the median duration was 7 days (range: 2-43 days).
CRS occurred in 84% (123/146) of patients with indolent non-Hodgkin lymphoma (iNHL) in ZUMA-5, including ≥ Grade 3 CRS in 8%. Among patients with iNHL who died after receiving YESCARTA, 1 patient had an ongoing CRS event at the time of death. The median time to onset of CRS was 4 days (range: 1-20 days) and median duration was 6 days (range: 1-27 days) for patients with iNHL.
Key manifestations of CRS (≥ 10%) in all patients combined included fever (85%), hypotension (40%), tachycardia (32%), chills (22%), hypoxia (20%), headache (15%), and fatigue (12%). Serious events that may be associated with CRS include, cardiac arrhythmias (including atrial fibrillation and ventricular tachycardia), renal insufficiency, cardiac failure, respiratory failure, cardiac arrest, capillary leak syndrome, multi-organ failure, and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS).
The impact of tocilizumab and/or corticosteroids on the incidence and severity of CRS was assessed in 2 subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received tocilizumab and/or corticosteroids for ongoing Grade 1 events, CRS occurred in 93% (38/41), including 2% (1/41) with Grade 3 CRS; no patients experienced a Grade 4 or 5 event. The median time to onset of CRS was 2 days (range: 1-8 days) and the median duration of CRS was 7 days (range: 2-16 days). Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA. Thirty-one of the 39 patients (79%) developed CRS and were managed with tocilizumab and/or therapeutic doses of corticosteroids with no patients developing ≥ Grade 3 CRS. The median time to onset of CRS was 5 days (range: 1-15 days) and the median duration of CRS was 4 days (range: 1-10 days). Although there is no known mechanistic explanation, consider the risk and benefits of prophylactic corticosteroids in the context of pre-existing comorbidities for the individual patient and the potential for the risk of Grade 4 and prolonged neurologic toxicities.
Ensure that 2 doses of tocilizumab are available prior to YESCARTA infusion. Monitor patients for signs and symptoms of CRS following infusion at least daily for 7 days at the certified healthcare facility, and for 4 weeks thereafter. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated.
NEUROLOGIC TOXICITIES
Neurologic toxicities including immune effector cell-associated neurotoxicity syndrome (ICANS) that were fatal or life-threatening occurred following treatment with YESCARTA. Neurologic toxicities occurred in 78% (330/422) of patients with NHL receiving YESCARTA, including ≥ Grade 3 in 25%.
Neurologic toxicities occurred in 87% (94/108) of patients with LBCL in ZUMA-1, including ≥ Grade 3 in 31% and in 74% (124/168) of patients in ZUMA-7 including ≥ Grade 3 in 25%. The median time to onset was 4 days (range: 1-43 days) and the median duration was 17 days for patients with LBCL in ZUMA-1. The median time to onset for neurologic toxicity was 5 days (range: 1-133 days) and median duration was 15 days in patients with LBCL in ZUMA-7. Neurologic toxicities occurred in 77% (112/146) of patients with iNHL, including ≥ Grade 3 in 21%. The median time to onset was 6 days (range: 1-79 days) and the median duration was 16 days. Ninety-eight percent of all neurologic toxicities in patients with LBCL and 99% of all neurologic toxicities in patients with iNHL occurred within the first 8 weeks of YESCARTA infusion. Neurologic toxicities occurred within the first 7 days of infusion in 87% of affected patients with LBCL and 74% of affected patients with iNHL.
The most common neurologic toxicities (≥ 10%) in all patients combined included encephalopathy (50%), headache (43%), tremor (29%), dizziness (21%), aphasia (17%), delirium (15%), and insomnia (10%). Prolonged encephalopathy lasting up to 173 days was noted. Serious events, including aphasia, leukoencephalopathy, dysarthria, lethargy, and seizures occurred. Fatal and serious cases of cerebral edema and encephalopathy, including late-onset encephalopathy, have occurred.
The impact of tocilizumab and/or corticosteroids on the incidence and severity of neurologic toxicities was assessed in 2 subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received corticosteroids at the onset of Grade 1 toxicities, neurologic toxicities occurred in 78% (32/41) and 20% (8/41) had Grade 3 neurologic toxicities; no patients experienced a Grade 4 or 5 event. The median time to onset of neurologic toxicities was 6 days (range: 1-93 days) with a median duration of 8 days (range: 1-144 days). Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA. Of those patients, 85% (33/39) developed neurologic toxicities; 8% (3/39) developed Grade 3, and 5% (2/39) developed Grade 4 neurologic toxicities. The median time to onset of neurologic toxicities was 6 days (range: 1-274 days) with a median duration of 12 days (range: 1-107 days). Prophylactic corticosteroids for management of CRS and neurologic toxicities may result in higher grade of neurologic toxicities or prolongation of neurologic toxicities, delay the onset, and decrease the duration of CRS.
Monitor patients for signs and symptoms of neurologic toxicities following infusion at least daily for 7 days at the certified healthcare facility, and for 4 weeks thereafter, and treat promptly.
REMS
Because of the risk of CRS and neurologic toxicities, YESCARTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS. The required components of the YESCARTA and TECARTUS REMS are:
- Healthcare facilities that dispense and administer YESCARTA must be enrolled and comply with the REMS requirements. Certified healthcare facilities must have on-site, immediate access to tocilizumab, and ensure that a minimum of 2 doses of tocilizumab are available for each patient for infusion within 2 hours after YESCARTA infusion, if needed for treatment of CRS.
Further information is available at www.YescartaTecartusREMS.com or 1-844-454-KITE (5483).
HYPERSENSITIVITY REACTIONS
Allergic reactions may occur with the infusion of YESCARTA. Serious hypersensitivity reactions, including anaphylaxis, may be due to dimethyl sulfoxide (DMSO) or residual gentamicin in YESCARTA.
SERIOUS INFECTIONS
Severe or life-threatening infections occurred after YESCARTA infusion. Infections (all grades) occurred in 45% of patients with NHL. Grade 3 or higher infections occurred in 17% of patients, including ≥ Grade 3 infections with an unspecified pathogen in 12%, bacterial infections in 5%, viral infections in 3%, and fungal infections in 1%. YESCARTA should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of infection before and after infusion and treat appropriately. Administer prophylactic antimicrobials according to local guidelines.
Febrile neutropenia was observed in 36% of patients with NHL and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
In immunosuppressed patients, including those who have received YESCARTA, life-threatening and fatal opportunistic infections including disseminated fungal infections (e.g., candida sepsis and aspergillus infections) and viral reactivation (e.g., human herpes virus-6 [HHV-6] encephalitis and JC virus progressive multifocal leukoencephalopathy [PML]) have been reported. The possibility of HHV-6 encephalitis and PML should be considered in immunosuppressed patients with neurologic events and appropriate diagnostic evaluations should be performed.
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, has occurred in patients treated with drugs directed against B cells, including YESCARTA. Perform screening for HBV, HCV, and HIV and management in accordance with clinical guidelines before collection of cells for manufacturing.
PROLONGED CYTOPENIAS
Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and YESCARTA infusion. Grade 3 or higher cytopenias not resolved by Day 30 following YESCARTA infusion occurred in 39% of all patients with NHL and included neutropenia (33%), thrombocytopenia (13%), and anemia (8%). Monitor blood counts after infusion.
HYPOGAMMAGLOBULINEMIA
B-cell aplasia and hypogammaglobulinemia can occur in patients receiving YESCARTA. Hypogammaglobulinemia was reported as an adverse reaction in 14% of all patients with NHL. Monitor immunoglobulin levels after treatment and manage using infection precautions,
antibiotic prophylaxis, and immunoglobulin replacement.
The safety of immunization with live viral vaccines during or following YESCARTA treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during YESCARTA treatment, and until immune recovery following treatment.
SECONDARY MALIGNANCIES
Patients treated with YESCARTA may develop secondary malignancies. T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA. Mature T cell malignancies, including CAR-positive tumors, may present as soon as weeks following infusion, and may include fatal outcomes.
Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
Due to the potential for neurologic events, including altered mental status or seizures, patients are at risk for altered or decreased consciousness or coordination in the 8 weeks following YESCARTA infusion. Advise patients to
refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 20%) in patients with LBCL in ZUMA-1 included CRS, fever, hypotension, encephalopathy, tachycardia, fatigue, headache, decreased appetite, chills, diarrhea, febrile neutropenia, infections with pathogen unspecified, nausea, hypoxia, tremor, cough, vomiting, dizziness, constipation, and cardiac arrhythmias.
INDICATIONMORE
YESCARTA® is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of:
- Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
Limitations of Use: YESCARTA is not indicated for the treatment of patients with primary central nervous system lymphoma.
Please see full Prescribing Information, including BOXED WARNING and Medication Guide.
Authorized Treatment Centers are independent facilities certified to dispense Kite CAR T therapies. Choice of an Authorized Treatment Center is within the sole discretion of the physician and patient. Kite does not endorse any individual treatment sites.
References: 1. Jacobson CA, Farooq U, Ghobadi A. Axicabtagene ciloleucel, an anti-CD-19 chimeric antigen receptor T-cell therapy for relapsed or refractory large B-cell lymphoma: practical implications for the community oncologist. Oncologist. 2020;25(1):e138-e146. 2. YESCARTA® (axicabtagene ciloleucel). Prescribing information. Kite Pharma, Inc; 2024. 3. Data on file [1]. Kite Pharma, Inc; 2023. 4. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas V.5.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed July 12, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. 5. Roberts ZJ, Better M, Bot A, et al. Axicabtagene ciloleucel, a first-in-class CAR T cell therapy for aggressive NHL. Leuk Lymphoma. 2018;59(8):1785-1796. 6. YESCARTA® and TECARTUS® REMS Patient Wallet Card. REMS-CTF-0026. April 2021. 7. Data on file [1]. Kite Pharma, Inc; 2024. 8. Data on file [2]. Kite Pharma, Inc; 2024. 9. Locke FL, Hemmer MT, Kanters S, et al. Axicabtagene ciloleucel vein-to-vein time in trial or real-world settings vs other CAR T-cell therapies for relapsed/refractory large B-cell lymphoma: a systematic literature review and meta-analysis. Presented at: 2023 European Hematology Association Meeting; June 8-11, 2023; Frankfurt, Germany. Poster 1204. 10. Data on file. Kite Pharma, Inc; 2023. 11. Data on file [3]. Kite Pharma, Inc; 2024. 12. Bansal R, Jonas P, Matthew H, et al. Outpatient practice utilization for novel immunotherapy in patients with lymphoma and multiple myeloma. Presented at: 2023 ASCO Annual Meeting; June 2-6, 2023; Chicago, IL. Abstract 1533. 13. Akosile TA, Dholaria BD, Mehraban N, et al. The impact of telemedicine and wearable devices in improving the safety profile of outpatient chimeric antigen receptor T-cell (CAR-T) therapies: a case-control study. Presented at: 2023 Transplantation & Cellular Therapy Meetings of ASTCT® and CIBMTR™; February 15-19, 2023; Orlando, FL.